JointAlive® is a specially formulated botanical blend with three traditional Chinese herbs: Epimedium brevicornum Maxim, Discorea nipponica Makino and Salvia miltiorrhiza Bunge. It is a safe solution for joint health, relieving joint pain and stiffness, in addition to increasing walking endurance to increase overall walking distance.
We conducted a 60-day, multi-center, open-label clinical study to prove the efficacy and safety of JointAlive® from March to July, 2020. The subjects aged between 60 and 75 years old with arthritis were enrolled. The knee arthritis was determined via X-ray scans with outcomes assessed by Western Ontario McMaster Universities Osteoarthritis Index (WOMAC) pain and stiffness scores at 7, 14, 21, 28, 45 and 60 days. Hematology, urine, blood pressure, and electrocardiogram measurements were collected before the study and at 30th day. The subjects took 600 mg JointAlive® daily for 60 days. During the study, 100 patients were enrolled and 98 patients finished the study.
The results showed JointAlive® can relieve joint discomfort quickly and with long lasting effects. 25% of patients felt less pain and stiffness within 7 days, whereas 46% experienced relief within 30 days (scores reduced more than 20%). 60% and 69% of patients reported joint comfort at days 45 and 60 respectively. (scores reduced more than 20%). The pain scores reduced from 6.71±2.43 at baseline to 4.14±2.04 on the 60th day (P<0.01). Stiffness scores reduced from 5.39±2.763 at baseline to 3.32±2.03 (P<0.01). Feedback suggested that JointAlive ® could also improve their waist, shoulder, and neck health.
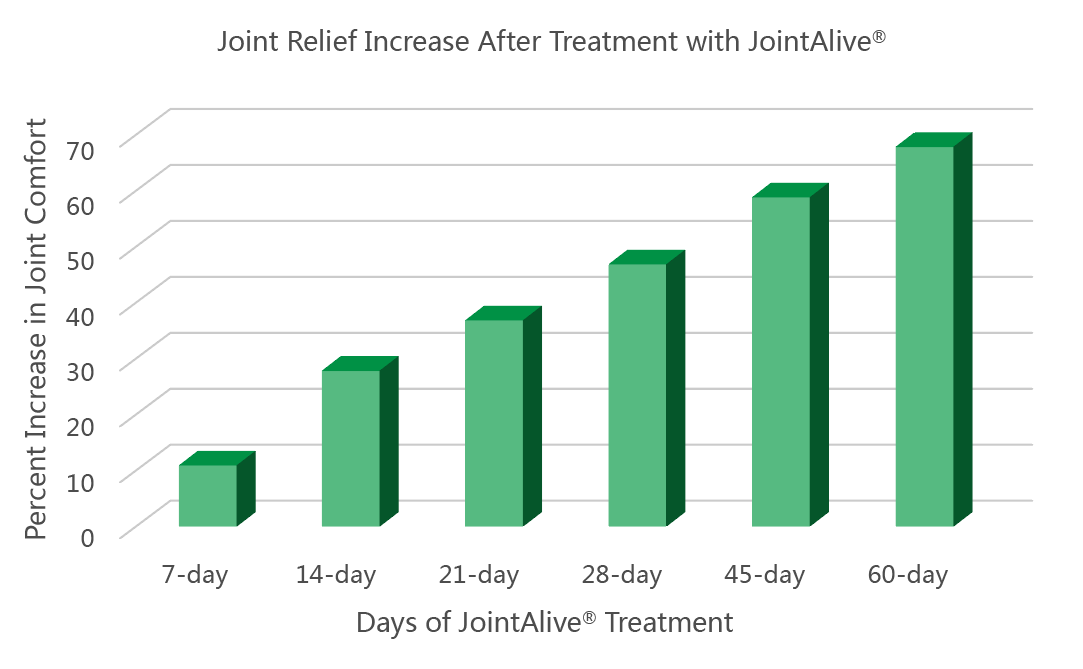
Fig 1 Patients number whose WOMAC scores reduced more than 20% in different visit day
Table 1 Score changes before and after treatment
Pain scores | Stiffness scores | |
Baseline | 6.71±2.43 | 5.39±2.763 |
60th day | 4.14±2.04** | 3.32±2.03** |
Compared to baseline data, **P<0.01
Hematology, urine, blood pressure and electrocardiogram results showed no significantly differences before and after the treatment, indicating the safety of JointAlive®.
Mechanism study
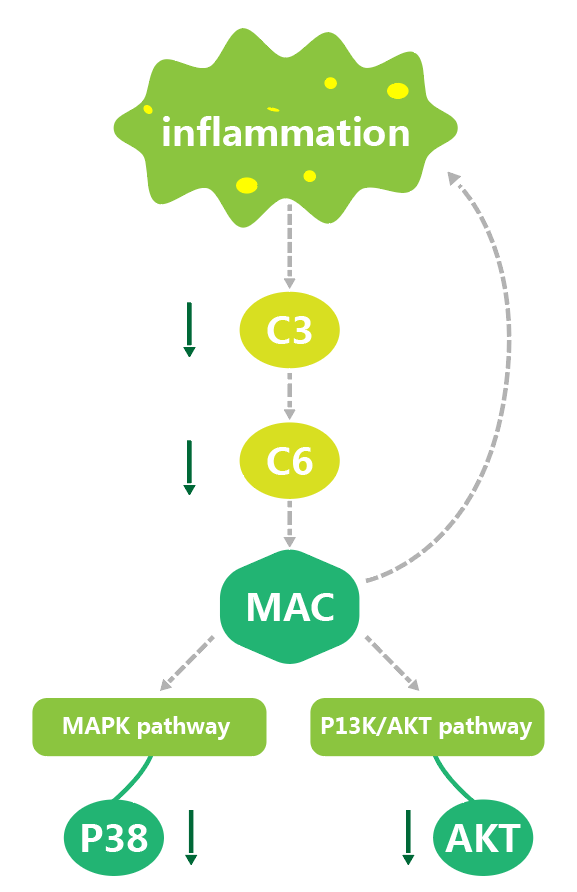
Chenland Nutritionals worked directly with the Pathology and Laboratory Medicine Department at Weill Cornell Medical College and found evidence that JointAlive® alleviates osteoarthritis through the modulation of protein expression. In-vivo preclinical studies showed that JointAlive® relieved symptoms from OA cartilage degradation, increased the threshold of the arthritis pain and reduced pain sensitivity, improved joint stiffness and increased joint flexibility, in addition to reducing the concentration of proinflammatory molecules in the serum of arthritic rats. Using proteomics and bioinformatics analysis, we found that the protective mechanism for JointAlive® is linked to depressing the abnormally activated complement system and increasing leukocyte mediated immunity.
We found that the complement C3 and C6 of OA group were significantly upregulated in both gene and protein levels. The levels of C3 and C6 in GA and ESD groups decreased to close to normal level. ApoE gene and protein level in the OA group increased significantly. Apolipoprotein ApoE level in GA and ESD group decreased to near normal level. Akt pathway related proteins Akt and p38 were upregulated in OA group in both gene and protein levels. Akt and p-p38 levels in GA and ESD groups were decreased to close to normal levels.
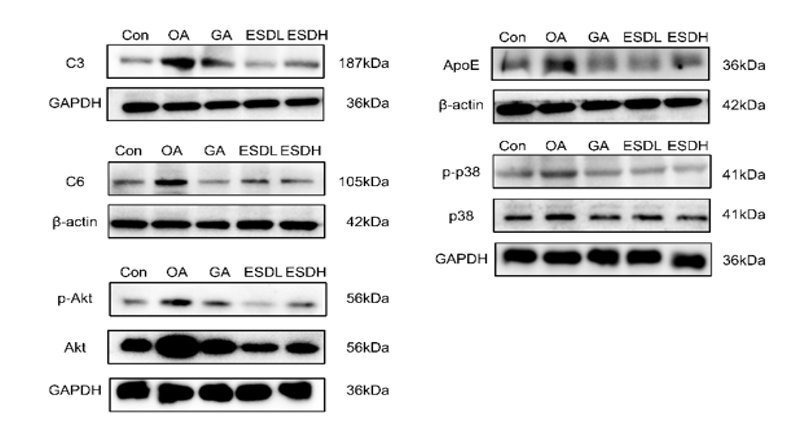
Con: Normal group;
OA: Placebo group;
GA: Glucosamine + Chondroitin Sulfate group
ESDL: JointAlive® Low-dose group
ESDH: JointAlive® High-dose group
Fig 2 PCR and WB verified the change proteins. The higher the protein expression, the more obvious it was on the electrophoretic diagram, indicating better treat effect on joint discomfort.
These overall findings provide evidence that JointAlive® activates the humoral immune response, by further activating MAPK, PI3K / AKT signaling pathways. It can down-regulate core protein factors, inhibit the response caused by inflammatory factors, and reduce joint pain inflammation to improve overall joint health.